Ever wonder why your pressure cooker transforms dried beans into tender perfection in under an hour when stovetop cooking takes hours? The secret lies in fundamental physics principles that dramatically accelerate cooking while enhancing flavor development. Understanding these mechanisms helps you harness this kitchen powerhouse safely and effectively.
The Core Science: Pressure and Temperature Relationship
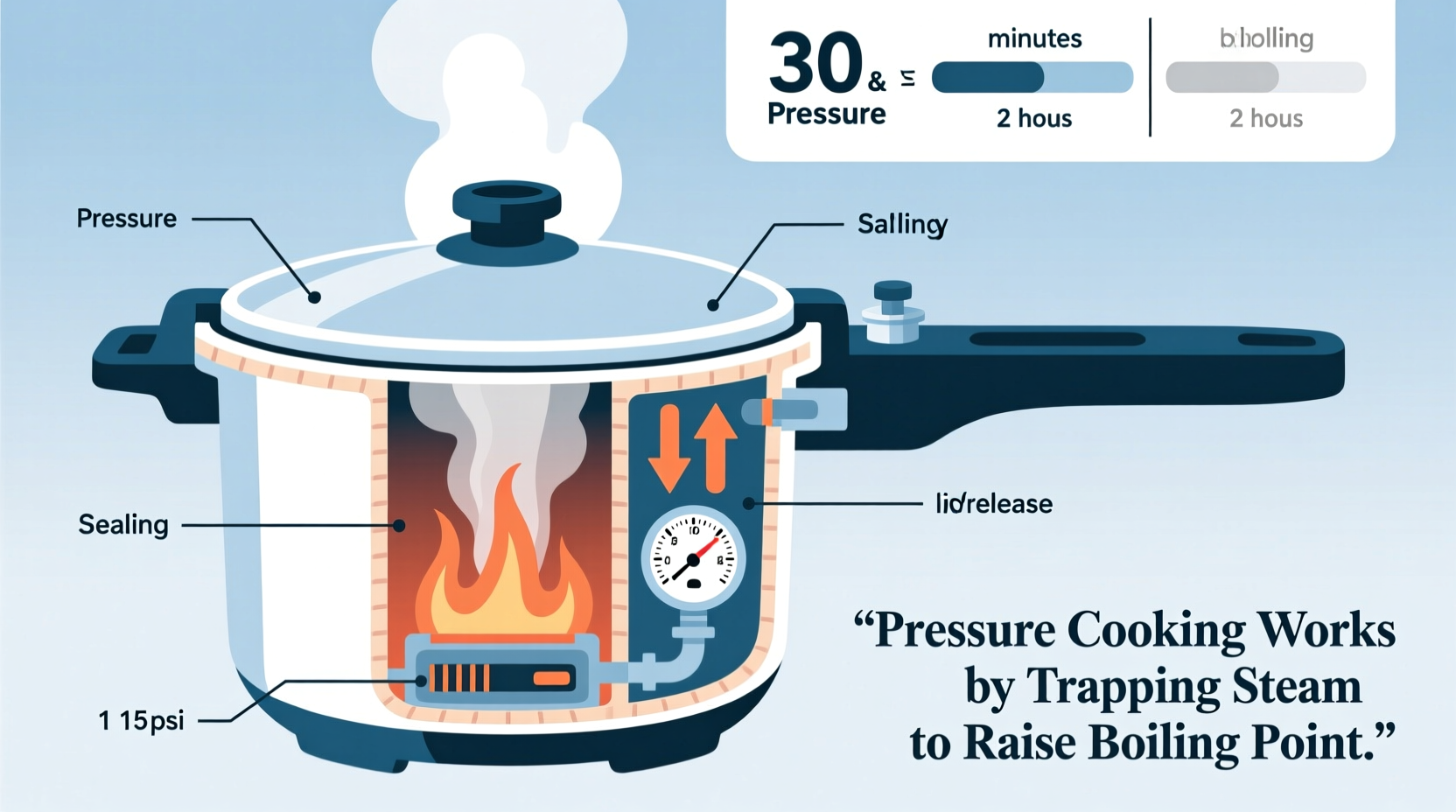
At sea level, water boils at 212°F (100°C) because that's when its vapor pressure equals atmospheric pressure. Pressure cookers create a sealed environment where steam can't escape, increasing internal pressure to typically 10-15 PSI above atmospheric pressure. According to the National Institute of Standards and Technology, this pressure increase raises water's boiling point to approximately 250°F (121°C).
| Pressure Level | Temperature | Cooking Speed vs. Boiling |
|---|---|---|
| Standard atmospheric | 212°F (100°C) | 1x (baseline) |
| 7 PSI (older models) | 230°F (110°C) | 2x faster |
| 12-15 PSI (modern) | 250°F (121°C) | 3-4x faster |
This temperature-pressure relationship follows the Clausius-Clapeyron equation from thermodynamics, which describes how a liquid's boiling point changes with pressure. Higher temperatures accelerate three critical cooking processes simultaneously: protein denaturation, starch gelatinization, and fiber breakdown.

How Steam Transfers Heat More Effectively
Steam carries approximately 2,260 kJ/kg of latent heat energy, making it a far more efficient heat transfer medium than air (which carries only about 1 kJ/kg·K). When steam contacts food surfaces inside the pressure cooker, it condenses back to water, releasing this substantial energy directly into the food. This explains why pressure cooking achieves thorough cooking without drying out foods, unlike oven methods where moisture evaporates into the air.
Safety Evolution: From Hazard to Household Essential
Early pressure cookers had significant safety concerns, but modern designs incorporate multiple fail-safes. Here's how safety mechanisms evolved:
| Era | Pressure Control | Safety Features | Incident Rate |
|---|---|---|---|
| 1930s-1950s | Weighted gauge | Single pressure release | High (1/1000 units) |
| 1960s-1980s | Spring-loaded valve | Backup fuse plug | Moderate (1/5000) |
| 1990s-Present | Precision spring valve | Triple safety system: overpressure plug, interlock lid, steam release | Very low (1/100,000) |
According to the U.S. Consumer Product Safety Commission, modern pressure cookers with these multiple safety systems have reduced accident rates by over 99% compared to early models. The interlocking lid mechanism prevents opening until pressure drops to safe levels, while redundant release valves ensure pressure never exceeds safe limits.
Practical Implications for Home Cooking
Understanding the physics translates directly to better cooking results. The elevated temperature accelerates the Maillard reaction (browning) while simultaneously breaking down collagen into gelatin. This dual action creates deeply flavored, tender results impossible with conventional methods. For example:
- Dried beans: Cook in 20-30 minutes instead of 2-3 hours
- Tough cuts of meat: Achieve fall-apart tenderness in 45-60 minutes
- Stocks and broths: Extract maximum flavor and nutrients in 2 hours
- Whole grains: Cook steel-cut oats in 3 minutes instead of 30
When Pressure Cooking Isn't Appropriate
While incredibly versatile, pressure cooking has specific limitations you should understand. The moist heat environment isn't suitable for:
- Foods requiring dry heat for proper texture (like baked goods)
- Dishes where ingredient separation is desired (like layered casseroles)
- Foods with high starch content that may clog valves (pureed pumpkin, thick oatmeal)
- Recipes requiring precise temperature control below 212°F
The University of Minnesota Extension notes that pressure cooking's minimum effective temperature (212°F at 0 PSI) makes it unsuitable for delicate tasks like tempering chocolate or cooking fish en papillote where lower temperatures preserve texture.
Optimizing Your Pressure Cooking Results
For best results, follow these evidence-based practices:
- Maintain proper liquid levels (at least 1 cup for most electric models)
- Natural release for fibrous foods (meats, beans) to complete cooking
- Quick release for delicate foods (vegetables, eggs) to prevent overcooking
- Always deglaze the pot after sautéing to prevent burn errors
- Reduce cooking times by 20-30% when converting stovetop recipes
Remember that pressure cooking continues during the natural release phase—up to 15 additional minutes of effective cooking time occurs as pressure drops gradually. This residual cooking is why many recipes specify a combination natural release followed by quick release.










 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4